Professor Hrant Hratchian's work published in JCP is chosen as an Editor's Pick! In this collaborative work with Dan Neumark's spectroscopy group at UC Berkeley, electron velocity-map imaging spectroscopy of cryogenically cooled TiO3H2− anions is used to reveal Herzberg-Teller coupling-induced transitions to Franck-Condon forbidden vibrational levels of the neutral ground state. The reaction with water stabilizes neutral TiO2 more than the anion, providing insight into the fundamental chemical interactions between titania and water.
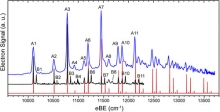
Titania (TiO2) is an inexpensive, extensively studied, and environmentally benign semiconducting material with widespread applications in photovoltaics, pollution management, chemical sensing, and heterogeneous catalysis. The landmark discovery of photosensitization of water on a TiO2 electrode sparked a decades-long pursuit to harness the photocatalytic properties of TiO2 as a practical means of solar-powered hydrogen fuel production. However, the success of this endeavor has been limited in part by a lack of the mechanistic understanding necessary to design better catalysts. Here, the researchers present high-resolution photoelectron spectra of the TiO3H2− anion accompanied by theoretical analysis, in order to probe the nature of the interaction between TiO2− and a single water molecule.



